Abstract

Background: Plasma levels of von Willebrand factor (VWF) and factor VIII (FVIII) increase dramatically during healthy pregnancy. These increases are markedly less pronounced in pregnancies affected by von Willebrand disease (VWD). Individuals who have VWD have high rates of primary and secondary postpartum hemorrhage (PPH), despite treatment. In 2021, guidance was published on the prophylactic use of tranexamic acid postpartum and target VWF levels for neuraxial anesthesia. Guidance is still lacking on VWF target levels for delivery and on the duration of treatment postpartum due to a lack of evidence.
Aims: To improve our understanding of bleeding during and after childbirth in individuals with VWD whose VWF levels are maintained >100%.
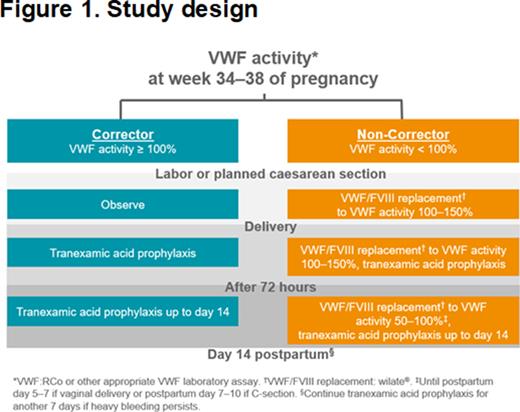
Methods: The VIP Study (NCT04146376) is an investigator-initiated, prospective, open-label, multicenter cohort study in the US designed to document the rate of PPH in VWD when VWF levels are maintained >100% during and after childbirth (Fig. 1). Pregnant patients ≥18 years of age and diagnosed with VWD type 1 (VWF ≤30%), type 2, or type 3 are eligible for enrollment. Patients whose third trimester VWF levels do not reach 100% are termed "non-correctors", while patients with third trimester VWF levels >100% are termed "correctors". Non-correctors will receive VWF/FVIII replacement therapy (wilate®) targeting VWF levels of 100-150% for delivery. VWF replacement will continue postpartum, targeting VWF levels of 100-150% through postpartum Day 3 and VWF levels of 50-100% thereafter until postpartum Day 5-10. All patients (non-correctors and correctors) will receive antifibrinolytic therapy (tranexamic acid) postpartum for 14 days. Target enrollment is completion of 65 non-correctors and up to 30 correctors. The primary outcome is the rate of PPH within the first 24 hours postpartum. Secondary outcomes include PPH from 24 hours to 6 weeks postpartum, laboratory assessment of coagulation, and safety, including adverse drug reactions and thrombotic events.
Results: As of March 2022, 11 sites across the US have been initiated and 9 patients have been enrolled.
Conclusion: The VIP study aims to address the knowledge gap regarding bleeding during and after childbirth in VWD. The data obtained will provide important information to help guide future steps to study and reduce PPH in VWD and to develop evidence-based management for these patients.
Disclosures
Johnsen:Octapharma: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Konkle:Spark: Honoraria; CSL Behring: Honoraria; BioMarin: Honoraria; Uniqure: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Sigilon: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Baxalta: Research Funding.
OffLabel Disclosure:
This study is investigating the use of a VWF/FVIII concentrate for the prevention of bleeds in patients with von Willebrand disease of any type.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal